Endovascular stent grafts are intended for the endovascular repair of fusiform aneurysms and saccular aneurysms/penetrating ulcers of the descending thoracic aorta. It helps reinforce the blood vessel and prevent an aneurysm from rupturing. Rupture of an aneurysm can result in internal bleeding and be fatal. It is used to relieve pressure on the damaged vessel wall.
[Figure 1- Normal Aorta vs. Aorta with large Aneurysm]
[Figure 2- Placement of Endovascular Stent Graft]
To insert the stent graft, the patient is put under general, regional, or local anesthesia. The patient lies on the operating table in the supine position to allow easy access to the femoral artery. An incision is made into this artery. The stent graft is compressed and loaded into a delivery system. This delivery system is a disposable catheter with a hydrophilic coating to ease the delivery of the stent graft. Using fluoroscopic guidance, the catheter is inserted through the femoral artery and carefully guided to the aneurysm. Once at the location of the aneurysm, the stent is deployed out of the delivery system and put into place. Once in place, the delivery system is carefully removed and the surgeon closes the incision near the patient’s femur.
Implantation:
1.The catheter is inserted into an artery in the leg near the groin.
2. Delivery catheter is inserted through the vessel into the aneurysm to guide the stent into place.
3. Using advanced imaging methods, the surgeon guides the delivery catheter carrying the stent graft to the area of the abdominal aortic aneurysm.
4. Once the stent graft is in position, the surgeon fastens it into place and removes the delivery catheter.
5. The endovascular stent graft is placed inside the abdominal aorta to help keep the aneurysm from bursting.
The current incidence of the disease refers to Aortic dissections which can be classified as Type A or Type B depending on where they occur. It is most commonly used to reinforce a weak spot in an artery referred to as an aneurysm.
- Type A aortic dissections begin at the ascending aorta, the segment closest to the heart.
- Type B aortic dissections begin in the descending aorta, may extend into the abdomen.
What is an Aneurysm?
Over time, blood pressure and other factors may cause the wall of an artery, capillary or vessel to bulge like a balloon and eventually enlarge or rupture.
What is a Stent Graft made from?
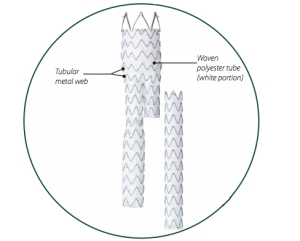
[Figure 3 – Components of a Stent Graft]
Polyester is most commonly used in endovascular stent grafts. It is very useful because of it’s high strength, resilience, lightweight, hydrophobicity, resistance to stretching and shrinking, abrasion resistance, and it’s ability to maintain heat-set pleats and creases.
The tubular metal web is typically made from nitinol (nickel-titanium). This metal web is used to give structural support to the stent graft. Nitinol has excellent corrosion resistance, a high fatigue strength, and it is biocompatible. [1]
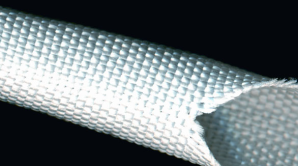
A woven structure is typically used because it is lightweight, has a high strength, and has a high flexibility and conformability. Because it is lightweight and flexible, it causes no discomfort to the patient. Like braids, the high strength is an advantage because it lasts the lifetime of the patient without being physically damaged. Conformability is also important because that is the ability for the product to conform to the shape of the artery. When the stent graft system is released from the catheter delivery system, it is important that it conforms to the shape of the artery so it can help the patient by decreasing the size of the bulge so that the aneurysm disappears. Also, it is important that it conforms easily so that it does not move out of place throughout the life of the patient. Since there are no sutures used to secure to the stent graft inside the patient, it is important that the device secures itself. It can be secured by oversizing the stent within the patient’s artery and by adding hooks to the proximal external surface of the stent graft to improve attachment to the abdominal aorta. [2]
[Figure 4 – An Example of a Woven Structure]
Other competitive biotextile options
Currently, there are no other biotextiles options for treating an aortic aneurysm. The endovascular stent graft is the only form of interventional repair. If the patient chooses not to treat the aneurysm with interventional repair, they can only watch and wait for it to repair itself or undergo open repair. The watchful waiting treatment is only recommended for patients who have an aneurysm that is smaller than 5 cm or 2 inches. For open surgical repair the surgeon sews a woven or knitted polyester (PET) graft into the position where the artery is cut open.
There are various types of endovascular stent grafts produced by different biotextile companies. Medtronic, W.L. Gore & Associates, Cook Medical and Terumo Vascutek are examples of companies that develop and produce the endovascular vascular stent grafts show below.
[Figure 5 – Variations of Endovascular Stent Grafts]
How is it prepared for implantation in the patient?
It is important to properly sterilize the endovascular stent graft before implanting it into a patient. The most commonly used method for sterilization is with Ethylene Oxide Gas. This is because it allows the sterilization of various materials. This is especially useful for a product like an endovascular stent graft because it is made up of various materials as well as a Nitinol layer that wraps around the outer surface. This method has been around for a long time and has a successful history. Because of it’s history, it is easy to see it is a reliable method of sterilization. The only disadvantage of Ethylene Oxide Gas that may negatively affect the endovascular stent graft would be that it has to be quarantined for 7-14 days. If surgeons do not have an adequate supply of sterilized endovascular stent grafts, the wait until they receive one could be very harmful to the patients health. However, with enough endovascular stent grafts on hand, the time the product is quarantined would not be a factor.
Surface Modifications
Because of the metallic web, endovascular stents are highly thrombogenic. This means that thrombus accumulates on the device and eventually leads to acute vessel occlusion. [4] A solution to this would be using hydrophilic polymers on the surface. These polymers would limit platelet adhesion and decrease reactivity. The polymers that can be used would be N-vinylpyrrolidone (NVP) and potassium sulfopropyl acrylate (KSPA). These are both very effective hydrophilic monomers that can be used to decrease the thrombogenicity.
To increase the haemocompatibility, the surface can be treated with inorganic coatings like hydrogenated amorphous carbon (a-C:H, frequently referred to as diamond-like carbon DLC). [5]
How is it tested?
Three in vivo animal studies were conducted to evaluate the early (up to 1 month) and late (up to 1 year) response to implantation of the FLAIR Endovascular Stent Graft and to evaluate the performance of the delivery system.[7] Two of these studies were conducted in canine iliac arteries and aortas to evaluate the performance of the first generation stent graft and delivery system. The third study was conducted in an intended Use canine model (arteriovenous access graft model) to evaluate the final stent graft design and delivery system. Subsequent to this animal study being conducted, process improvements were made to the stent graft to optimize fatigue resistance and manufacturability.[7] These modifications were evaluated through in vitro testing, which confirmed device equivalence; therefore, additional animal testing was not warranted. All studies were conducted in accordance with FDA Non-Clinical Good Laboratory Practice Regulation 21 CFR, Part 58.[7]
What are some Complications?
Leaking of blood around the graft, infection, movement of the graft away from the desired location, graft fracturing, blockage of the blood flow through the graft. Sometimes fever and an increase in white blood cell count can happen shortly after endovascular stent grafting.
[Figure 6 – Migration of a Stent Graft]
Suggestions: One idea that membrane-coated stents might improve the prognosis in patients with subacute/chronic type B dissection has been proposed.
Currently, the FLAIR Endovascular Stent Graft has not been marketed in the United States or any foreign country. The endovascular stent-graft market will continue to expand rapidly for a number of reasons. Increasing numbers of patients will select this less-invasive procedure over the traditional open surgery with its longer recovery periods and higher rates of complications. In addition, the aging population will continue to expand, putting more people at risk for aneurysm.
Business Strategy:
*The decision on whether endovascular repair is preferred over open surgical repair should be made jointly by the patient and their clinician after assessment of a number of factors including:
- aneurysm size and morphology
- patient age, general life expectancy and fitness for open surgery
- the short-term and long-term benefits and risks of the procedure including aneurysm mortality and operative mortality.
- Primary Market (End User)– Middle Aged- Senior Citizens. Male/Female patients, specifically around age 50-78, with current cardiovascular health related issues. Three times more common in Men than in Women however.
- Secondary Market- Surgeons, Cardiologists, Hospitals.
- Basis of Product-less invasive procedure, covered by insurance. *Not many other options for treatment*
- 88% success rate of effectively performed operation.
-
can cost up to around $8,000-$16,000; but can be dependent on the person and number of accessories used in aid of procedure.
References
[1] Nickel-Titanium Shape Memory Alloys
[2] Woven Structures
[3] Aneurysms
[4] Hydrophilic Surface Modification
[5] Haemocompatibility
[6] Appraisal Consultation Document
[7] FDA Summary of Safety and Effectiveness Data
[Final Version, edits & corrections received from comments have been completed ]





The overall layout of the blog is good; but towards the end add some more pictures or diagrams to help break up the words.
Very organized information with great use of pictures.Great Job!
The information you gave was very informative and was presented in a understandable order. Great job.
I agree
Great headings! The report is very well organized. Encompassing without being too lengthy. Very nice.
Excellent images and video showing deployment of the stent-graft in a AAA site. This is very helpful. The section entitled “What is an Aneurysm” needs to refer to the wall of the artery, vein, capillary or vessel!! In the section on “Other Competitive Biotextile Options”, you refer to “surgical repair”, by which I assume you mean “open repair”. For “open surgical repair” the surgeon sews a woven or knitted polyester (PET) graft into the position where the artery is cut open. The term “plastic graft” is not appropriate, given this is a course on biotextile products!!
Also please note that while C.R. Bard manufactures small diameter vascular grafts (Venaflo II), it does NOT manufacture or distribute an endovascular stent-graft product!! Medtronic, W.L. Gore & Associates, Cook Medical and Terumo Vascutek do!! Please avoid misleading claims on your blog. Thanks.
The use of the term “metallic web” is not very clear in the “Surface Modification” section. The metallic stent can be assembled on the inside or on the outside of the woven polyester fabric or ePTFE membrane tubular graft material. Polyester is highly thrombogenic, while ePTFE is non-thrombogenic!!
I like your section on “How is it tested?” This section refers to the specific in vitro and in vivo test protocols for a particular manufacturer’s device called the Flair stent-graft. Can you give the reader a reference where the details of this study are explained? Your five linked references work well!! You could include the “Appraisal Consultative Document” and other sources mentioned above as additional references!!
Please check for typographical errors (e.g. Endovascular Stent-Grafts, 3rd line: “thoracic aorta”; How is it prepared for implantation in the Patient?, 3rd line: “because it”; Surface Modifications, 7th line: “effective very effective”.) Thanks alot. MWK
Great Information about Endovascular Stent Grafts. Keep posting
We are at Healing Touristry an ISO Certified 9001:2015 Indian healthcare service provider that offers medical treatment to foreign nationals, seeking affordable and safe medical tourism facilities… Click here https://www.healingtouristry.com/